By Xiaoteng Gong, Dujuan Lu, Danny Hower, Julian Gulbinski, Daniela Cavagnino, and Cristian Cojocariu.
Optimizing Headspace GC-MS Analysis for Extractables and Leachables in Pharmaceuticals
Pharmaceutical products encounter various polymeric materials during their lifecycle, from manufacturing and storage to administration. These interactions can lead to the leaching of potentially harmful substances into the drug products, posing safety risks. A recent study published by Thermo Fisher Scientific addresses this concern by optimizing headspace gas chromatography-mass spectrometry (GC-MS) methods for analyzing volatile extractables and leachables (E&L).
Understanding the Study
The study aimed to improve the detection and identification of volatile impurities using headspace GC-MS, a technique well-suited for analyzing the volatile fraction of E&L compounds. By optimizing sampling strategies, including incubation temperature, salt addition, and sample/vial volume ratio, the researchers enhanced the sensitivity and selectivity of the method.
Key Findings
- Optimization of Parameters: The study focused on optimizing several parameters to improve extraction efficiency. These included incubation temperature, sample volume, and the addition of salt to favor the concentration of volatile compounds in the headspace.
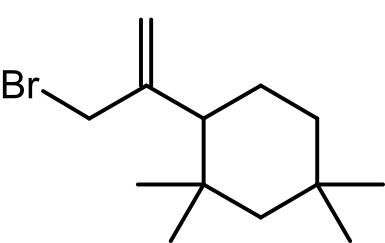
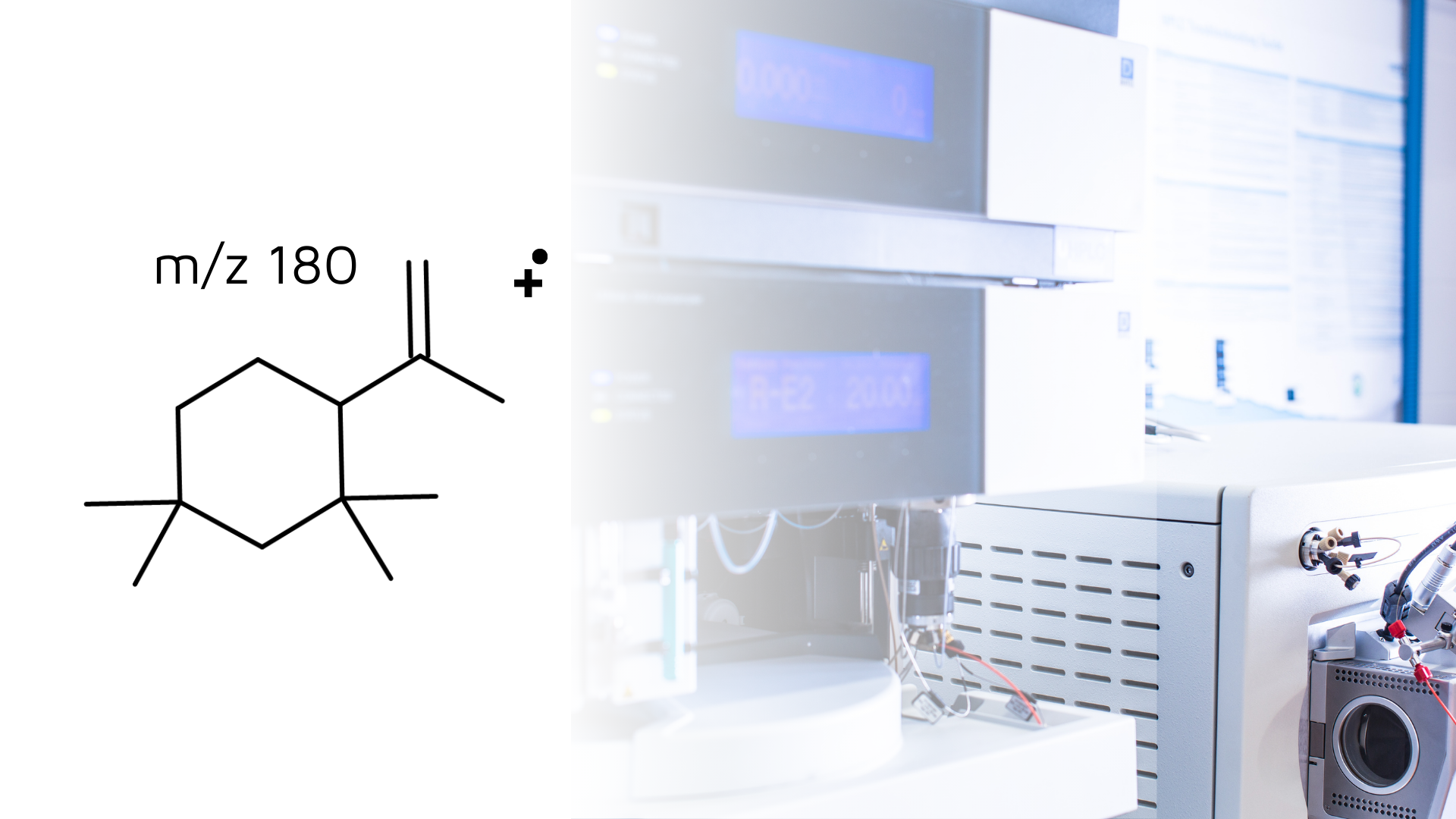
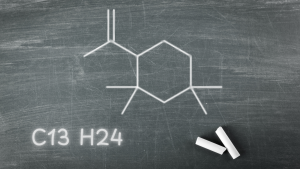
- Identification of C13H24: A significant finding was the identification of the rubber oligomer C13H24, a typical impurity formed during the copolymerization of isoprene and isobutylene. This oligomer was detected in the dry solid sample headspace but not in the water extract, highlighting the importance of using appropriate extraction conditions.
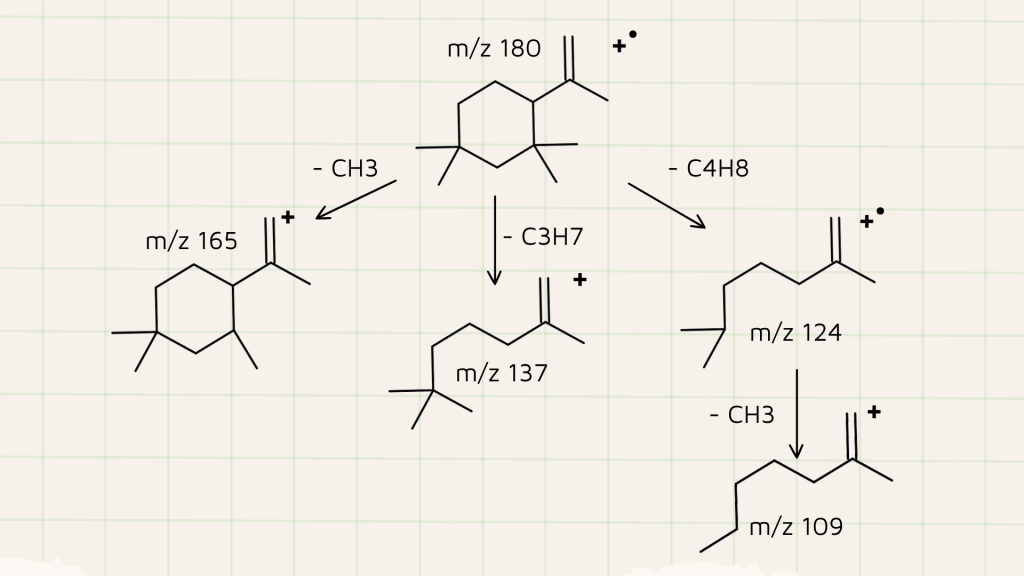
- Mass Spectrometry Analysis: The mass spectrometry analysis revealed several key fragments of C13H24 that helped elucidate its structure. These fragments include m/z values of 180, 165, 137, 124, and 109, which correspond to C13H24, C12H21, C10H17, C9H16, and C8H13, respectively. The accurate mass measurements enabled a confident identification of these fragments, providing a detailed understanding of the compound’s structure.
Conclusion
The optimized headspace GC-MS method developed in this study offers a robust solution for profiling and identifying volatile extractables and leachables in pharmaceutical products. By improving the sensitivity and selectivity of the analysis, this method helps ensure the safety and quality of pharmaceuticals.