By Piet Christiaens and Lothar Habel
https://vdoc.pub/download/extractables-and-leachables-europe-2012-3qjdd3nhhkf0
The safety of pharmaceutical products relies heavily on understanding the materials used in their packaging. A study presented by Piet Christiaens and Lothar Habel at the Extractables & Leachables 2012 conference sheds light on the analysis of (halo)butyl oligomers in pharmaceutical rubbers. These materials, commonly used in closures like stoppers and syringe plungers, can leach potentially harmful compounds into drug products.
Key Findings
1️⃣ Identification of Oligomers:
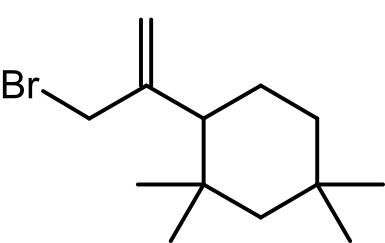
The study focused on detecting and characterizing oligomers such as C13H24, C13H23Cl, C13H23Br, and C21H40 in halobutyl rubbers. These compounds often appear as unknowns in mass spectra due to their absence from commercial spectral libraries.
2️⃣ Mass Spectrometry Analysis:
The research employed GC-MS and headspace GC-MS to identify key fragmentation patterns of these oligomers. For instance:
- C13H24 produces a mass peak at m/z 68.
- C13H23Cl yields peaks at m/z 102/104.
- C13H23Br shows peaks at m/z 146/148.
- C21H40 produces a peak at m/z 180.
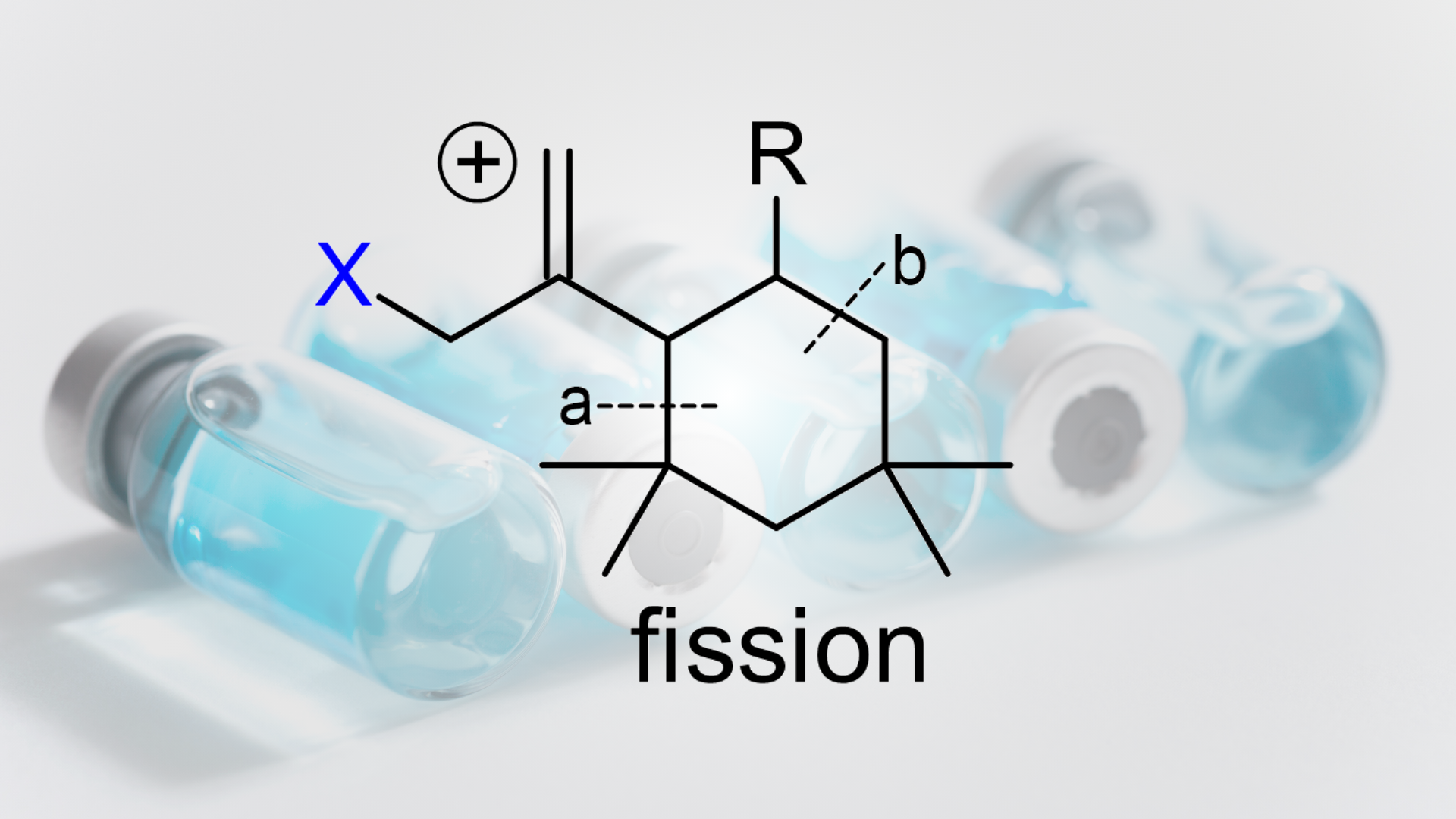
These fragmentation patterns were explained by a common a/b fission fragmentation mechanism, providing critical structural insights.

3️⃣ Formation Mechanism:
The study proposed that protonation of isoprene initiates the formation of these oligomers, followed by the addition of isobutylene and cyclization. This process results in stable six-membered ring structures, explaining the stability and formation preferences of C21H40 over related compounds like C17H32, which cannot form through the same pathway.
4️⃣ Toxicological Assessment:
A Structure Activity Relationship (SAR) assessment, using Derek Nexus software, evaluated the toxicity risks of these oligomers. While non-halogenated oligomers like C13H24 were classified as relatively low risk, halogenated oligomers such as C13H23Cl, C13H23Br, C21H39Cl, and C21H39Br were flagged for potential carcinogenicity. These compounds are categorized as Allyl Halides and Alkylating Agents, both of which raise concerns due to their DNA-damaging potential.
Due to limited toxicological data, many pharmaceutical companies classify these halogenated oligomers as high toxicological concern, underscoring the need for further studies to assess their safety comprehensively.
Implications for Pharmaceutical Safety
The detection and structural elucidation of (halo)butyl oligomers are essential for assessing the safety of pharmaceutical products. This study provides valuable insights into how these compounds form and behave, guiding efforts to better control potential leachables in packaging systems.
By combining advanced analytical methods with toxicological evaluations, this research contributes significantly to improving pharmaceutical safety and ensuring drug integrity.
For a deeper dive into the intricate details of this study, refer to the full article presented at the Extractables & Leachables 2012 conference by former Toxikon Europe—nowadays Nelson Labs.
https://vdoc.pub/download/extractables-and-leachables-europe-2012-3qjdd3nhhkf0