By Fabrizio Minicone, Robin Attrill, Michael Hodgson, Katherine Wheelhouse, and Adrian Dobbs
European Journal of Organic Chemistry – doi: 10.1002/ejoc.201800496
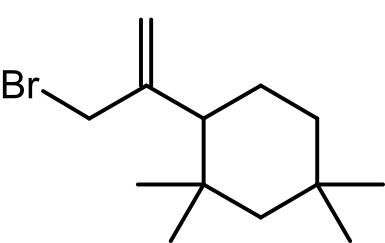
Elastomeric closures, such as stoppers and caps, play a critical role in pharmaceutical packaging by maintaining drug sterility and integrity. However, these materials can introduce impurities, such as C13H24 and its halogenated derivatives, into drug products, raising safety concerns.
A study by Fabrizio Minicone and colleagues, published in the European Journal of Organic Chemistry, presents the first-ever scalable synthesis and structural confirmation of a C13-butyl rubber oligomer. This achievement addresses the need for a reliable method to produce these compounds for toxicity studies and safety assessments.

Key Findings
- Innovative Synthesis: The research team developed a robust, reproducible synthesis starting from isophorone. Key steps included dithoacetal chemistry, cuprate addition, and Tebbe olefination, culminating in the production of the C13 oligomer.
- Structural Confirmation: NMR and GC-MS analyses confirmed that the synthesized oligomer matched its naturally extracted counterpart. Characteristic NMR peaks at δ = 4.64 and 4.82 ppm validated the terminal alkene structure.
- Scalability: The method enables the production of sufficient quantities of the oligomer for AMES toxicity testing, marking a significant step forward in understanding the potential health risks posed by these compounds.
Implications
This research bridges the gap between synthetic chemistry and pharmaceutical safety, providing a pathway for evaluating and mitigating the risks associated with extractables and leachables in medical materials.
For a detailed exploration, consult the full study in the European Journal of Organic Chemistry.