by Richard Jähnke, Jörg Kreuter, and George Ross
Rubber closures are vital in pharmaceutical packaging, serving as a barrier to protect drugs from contamination and maintain sterility. However, a study published in the Journal of Parenteral Science and Technology reveals that these closures can leach volatile organic compounds (VOCs), posing potential risks to drug safety.
Key Findings
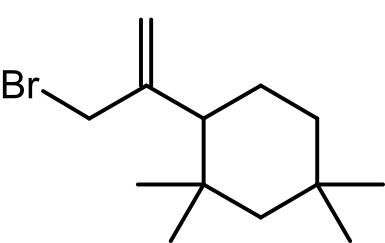
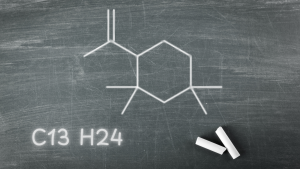
- Volatile Organic Compounds (VOCs): The study identified hydrocarbons, including chlorinated derivatives like C13H23Cl, that can migrate from rubber stoppers into injectable powders. These compounds were detected using GC-MS and confirmed through NMR spectroscopy.
- GC-MS Analysis: Chlorinated oligomers were identified with mass signals at m/z 214 and 216, while non-chlorinated counterparts showed distinct fragmentation patterns. These analyses highlight the complexity of VOC contamination.
- NMR Insights: The chlorinated oligomer C13H23Cl exhibited characteristic peaks at δ = 4.97 ppm and δ = 5.31 ppm, providing further evidence of its chemical structure and presence.
- Temperature Effects: Temperature played a critical role in the detection of these contaminants. Non-chlorinated oligomers appeared at 123°C, while chlorinated species were detected at 160°C, simulating conditions encountered during sterilization and drug storage.
Implications for Pharmaceutical Safety
This study underscores the need for rigorous quality control in pharmaceutical packaging materials. Understanding how rubber closures interact with drug formulations is crucial for ensuring the safety, efficacy, and stability of injectable products.
Conclusion
By shedding light on the interactions between rubber stoppers and injectable powders, this research provides valuable insights for the pharmaceutical industry. Implementing these findings can help mitigate contamination risks and enhance drug safety.